MR Spectroscopy & Ultra-High Field Methodology
Shortly after introduction of in vivo magnetic resonance imaging (MRI) also first localized in vivo magnetic resonance spectra (MRS) were acquired in the early 1980s. In vivo MRS has evolved during the last 30 years in terms of localization quality and spatial resolution, acquisition speed, artifact suppression, number of detectable metabolites and quantification precision and has profited especially from the significant increase of magnetic field strength that recently became available for in vivo investigations. Today it allows for non-invasive and non-ionizing determination of tissue concentrations and metabolic turn-over rates of various metabolites and compounds in animals or humans, is applied for clinical diagnostics and has established as an important tool for physiological research.
Magnetic Resonance Spectroscopy Methodology

To draw physiologically meaningful conclusions spectroscopy results need to be spatially and metabolically specific and all confounding factors related to acquisition, reconstruction and quantitative analysis need to be considered and artifact sources controlled to obtain reliable and reproducible results. To that a high localization accuracy and efficiency including coherence pathway selection and motion compensation methods need to be developed. Calibration steps such as flip angle optimization or B0 shimming have to be robust. Quantification has to be based on spectral fitting algorithms exhibiting robust convergence and include comprehensive prior-knowledge and stable reference standards. In addition, acquisition times need to be shortened by different acceleration techniques for clinical use, spectroscopic imaging with whole organ coverage at high spatial resolutions and functional magnetic resonance spectroscopy.
Current projects of specific interest include
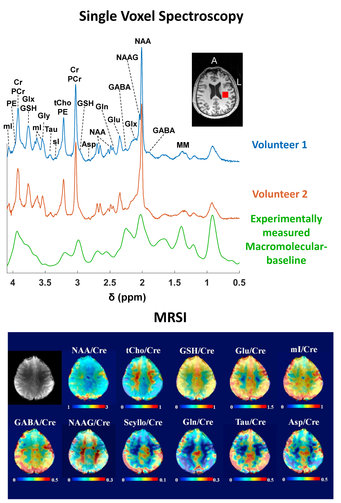
- spectroscopy localization
- accelerated MRSI and related MRSI reconstruction
- functional 1H and 13C MRS
- 31P MRS and MRSI
- motion correction methods for body MRS (spinal cord, myocardium)
- 2D resolved and edited MRS
- spectral fitting
- ERETIC reference standard
- Absolute quantification for MRSI
Ultra-high field magnetic resonance methodology
The higher the static magnetic field strength the higher are spectral separation and signal-to-noise ratio, which results in a largely increased number of detectable metabolites at high spatial specificity at ultra-high field strength. However, the advantages of high (3T) and ultra-high (7T) field strength come along with extensive technical challenges such as inhomogeneous transmit (B1) fields due to standing wave effects, an increased impact of microscopic and macroscopic susceptibility differences on static magnetic field (B0) inhomogeneity, shortened T2 and lengthened T1 relaxation times, lower effective B1 field strength and scan time prolongation due to specific-absorption-rate restrictions. In addition respiratory motion induces time dependent B0 field fluctuations which need to be addressed to extend the applicability of in vivo MRS to a larger number of organs. The aim of our ultra-high field methods development projects is hence to overcome these technical challenges in order to extend the number of quantifiable metabolites and to increase spatial resolution and hence specificity. In general related methodology can be transferred to additional MR imaging modalities.
Current projects of specific interest include
- radiofrequency pulse design
- parallel transmit and receive technology (RF coil arrays, EM simulation and optimization)
- real-time feedback, dynamic, higher order B0 shim technology
Multimodal high-field MRS and MRI for physiological research
Newly developed magnetic resonance spectroscopy methodology may enable a more profound understanding of healthy and pathological physiology. Hence these methods are evaluated with respect to their relevance for clinical diagnostics and combined with structural and functional MRI modalities such as high resolution anatomical imaging, resting-state functional connectivity imaging, perfusion imaging, diffusion weighted imaging or complementary non-MR imaging methodology such as PET for physiological or pathophysiological studies. Physiological studies usually require assessment of additional biomarkers and may benefit from a translational approach in humans and animals. Physiological studies are typically joint projects with collaborators with physiological or clinical background and/or expertise in a complementary imaging method.
Current physiological studies focus on the following topics:
- Psychiatric Disorders: major depressive disorder and mechanism of action of related therapy forms
- Neurological Disorders: spinal cord pathology and regeneration
- Muscle physiology: skeletal and myocardial muscle energy metabolism in metabolic syndrome